JBA GlucoTrojan® White Paper
The power of mitigating blood sugar spikes in enhancing our health
By Guillermo Daniel Repizo, PHD; Shin Jie Yong; Le Quang Huan, PHD
October 21, 2023
Summary
Researchers have recently coined the term “diabesity,” the combined diabetes and obesity epidemics for what they think it could be the greatest health crisis in human history. Indeed, our modern diet, abundant in carbohydrates and sugars, often causes surges in blood glucose levels that could spiral into a vicious cycle of increased insulin production and insulin resistance. Excess insulin promotes unnecessary fat storage, thus leading to obesity, whereas insulin resistance impairs glucose uptake, paving the way for diabetes.
This escalating health crisis has propelled the innovation of GlucoTrojan®, a synergistic blend of white mulberry leaf, mango leaf, and banana stem juice extracts. Designed with scientific precision, GlucoTrojan® helps optimize glucose metabolism and insulin sensitivity via a simple mechanism: mitigating excessive spikes in postprandial (after meals) blood glucose levels.
Scientific data, including randomized clinical trials and our real-world observational study led by Tastermonial, have consistently demonstrated the efficacy of GlucoTrojan® components in improving postprandial blood sugar levels and insulin response. A well-regulated blood glucose response promotes satiety, prevents cravings, enhances cellular energy metabolism, and consequently delays aging. GlucoTrojan® is, therefore, a promising supplement for those with prediabetes and those seeking to improve their health and well-being.
The Greatest Health Crisis in Human History
There has never been an easier time to secure food than today, particularly the rich, calorie laden foods our ancestors would have deemed precious gold. With our survival instincts remaining largely intact, it is no surprise we have an epidemic of “diabesity,” a sinister fusion of type 2 diabetes and obesity [1]. About 11% and 42% of the U.S. population suffers from
diabetes and obesity, respectively, in 2022 [2, 3]. Several studies support the hypothesis that diabesity is the largest epidemic and health crisis in human history [1]. Are we ready to face it?
Before diving into promising solutions, we must first understand what drives the diabesity epidemic. While genetics play a significant role in our predispositions, lifestyle factors (e.g., physical inactivity and poor nutrition) remain the leading drivers [4]. Regarding nutrition, reports indicate that excess sugars top the list of causes of diabetes and obesity, due to the direct influence of sugars on insulin levels [5].
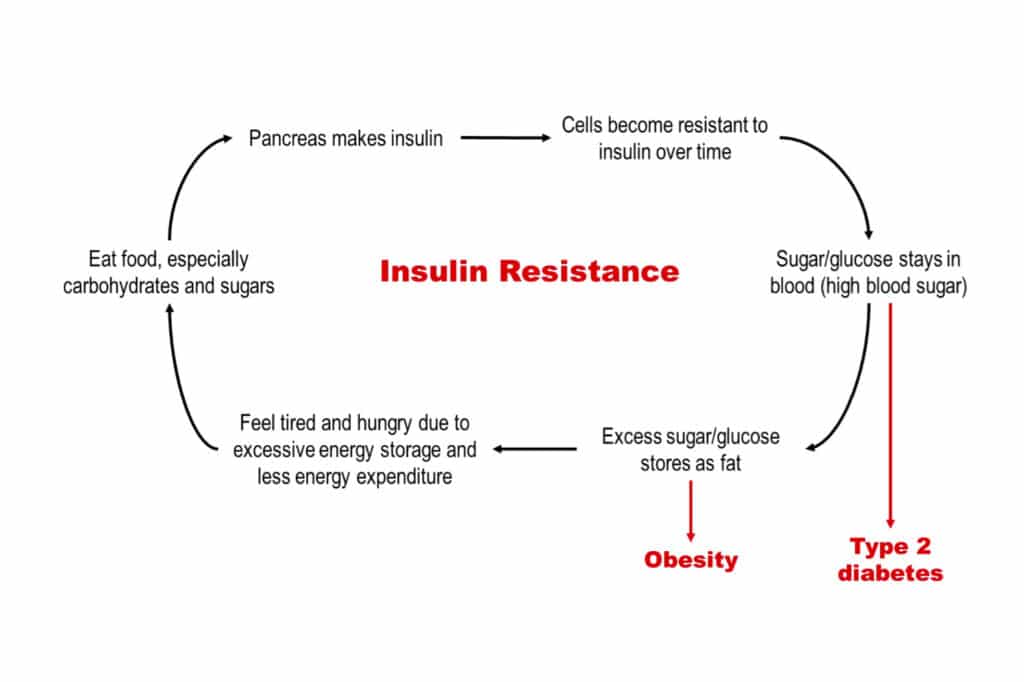
Diabetes and obesity are diseases fundamentally caused by insulin resistance, a state in which cells become unresponsive to insulin (i.e., a hormone that enables cells to absorb glucose, with the surplus being converted to fat). To overcome this resistance, the pancreas ramps up insulin production. However, excess insulin induces fat storage and then obesity, whereas the persistently high blood sugar levels, due to ineffective glucose uptake, promotes diabetes (Fig 1) [6]. An additional 7.3% of the general population is estimated to have insulin resistance [7].
Importantly, insulin resistance does not immediately cause diabetes and obesity. It unfolds progressively, with cells becoming incrementally more resistant to insulin and prone to storing fat, largely driven by the excess sugar in our modern diet [8]. If we address the root cause of insulin resistance – excess sugar – we can chart a course toward a healthier life-style.
JBA GlucoTrojan®: A Solution to Healthier Glucose Metabolism
Let us introduce JBA GlucoTrojan®, an innovative powdered supplement with the main active ingredient being white mulberry leaf extract. Interestingly, mulberry leaf contains iminosugars, whose molecular structure is similar to common sugars, but with the special property that they can influence enzymes involved in carbohydrate metabolism.
For instance, a key iminosugar, 1-deoxynojirimycin (DNJ), is a potent inhibitor of α glucosidase enzymes that aid carbohydrate assimilation in the gut [9]. DNJ, thus, mitigates glucose absorption in the intestine and accelerates liver metabolism of sugar to increase energy expenditure [10]. In short, DNJ propels the body into fat-burning mode by consuming more energy.
Rigorous randomized clinical trials (RCTs) across multiple countries have demonstrated that mulberry extract, rich in DNJ, is safe and efficacious in reducing postprandial (post-meal) blood sugar and insulin levels compared to control in healthy individuals and even individuals with insulin resistance, obesity, or diabetes (Table 1). In medicine, RCTs are considered the gold-standard method for determining the efficacy of certain interventions.
Table 1. Randomized clinical trials examining the efficacy of DNJ-containing mulberry extract (ME) in controlling blood sugar and insulin responses.
| Study | Sample | Intervention | Main outcomes |
| Mudra et al.
(2007), U.S. [11] |
10 patients with
diabetes: aged 59-74. |
1000 mg of ME containing DNJ vs. placebo | ↓ blood glucose levels over 120 min after
drinking sucrose solution. |
| Asai et al.
(2011), Japan [12] |
10 participants with prediabetes: mean age of 50; 80% men. | 6-9 mg of DNJ vs. placebo | ↓ blood glucose levels at 30 min after eating white rice. |
| 65 participants with prediabetes: mean age of 53; 66% men. | 6 mg of DNJ vs. placebo | ↑ blood 1,5AG level
(glycemic control biomarker) when consumed with meals over 12 weeks. |
|
| Lown et al.
(2017), U.K. [13] |
37 healthy
participants: mean age of 29; 32.4% men. |
250-500 mg of
ME containing 5% DNJ vs. placebo |
↓ blood glucose and
insulin levels over 120 min after drinking maltodextrin solution. |
| Riche et al.
(2017), U.S. [14] |
24 patients with
diabetes: mean age of 57; 42% men. |
1000 mg of ME containing DNJ vs. placebo | ↓ blood glucose levels when consumed with meals over 12 weeks. |
| Wang et al.
(2018), China [15] |
15 healthy
participants: mean age of 24; 60% men. |
750 mg of ME
containing 1% DNJ vs. no extract |
↓ blood glucose levels at 30 min after drinking water containing sucrose, maltose, and
maltodextrin. |
| Thaipitakwong a et al. (2019), Thailand [16] | 85 individuals with obesity: mean age of 24; 20% men. | 4600 mg of ME containing 0.25% of DNJ vs. no
extract |
↓ blood glucose and
HbA1c levels when consumed with meals over 12 weeks. |
| Thondre et al. (2021), U.K.
[17] |
38 healthy
participants: mean age of 32; 42% men. |
250 mg of ME
containing 5% DNJ vs. placebo |
↓ blood glucose and
insulin levels over 120 min after drinking sucrose solution. |
| Gheldof et al. (2022),
Switzerland [18] |
30 healthy
individuals: mean age of 31; 63% men. |
250mg of ME
containing 5% DNJ vs. no extract |
↓ blood glucose levels at 2 hours after meal. |
Abbreviations: 1,5AG, 1,5-anhydroglucitol; DNJ, 1-deoxynojirimycin; HbA1c, glycated hemoglobin; ME, mulberry extract.
RCTs, however, often recruit participants with similar characteristics (e.g., age, sex, and health status) to maximize the replicability of results. Therefore, real-world observational studies are also needed to extrapolate results derived from the strict laboratory settings of RCTs to the highly diverse general population [19].
Tastermonial Inc, a company specialized in real-world data obtention, conducted a study which provided further scientific evidence on the health benefits of GlucoTrojan® in 25 healthy individuals with prediabetes. A state of prediabetes indicates sub-clinical insulin resistance, which may develop into clinical diabetes if left unattended [20]. Our eligibility criteria ranged from slim to obese (BMI of 18.5 to 29.9) and young to old (21-75 years) individuals, ensuring a broad representation of the general population.
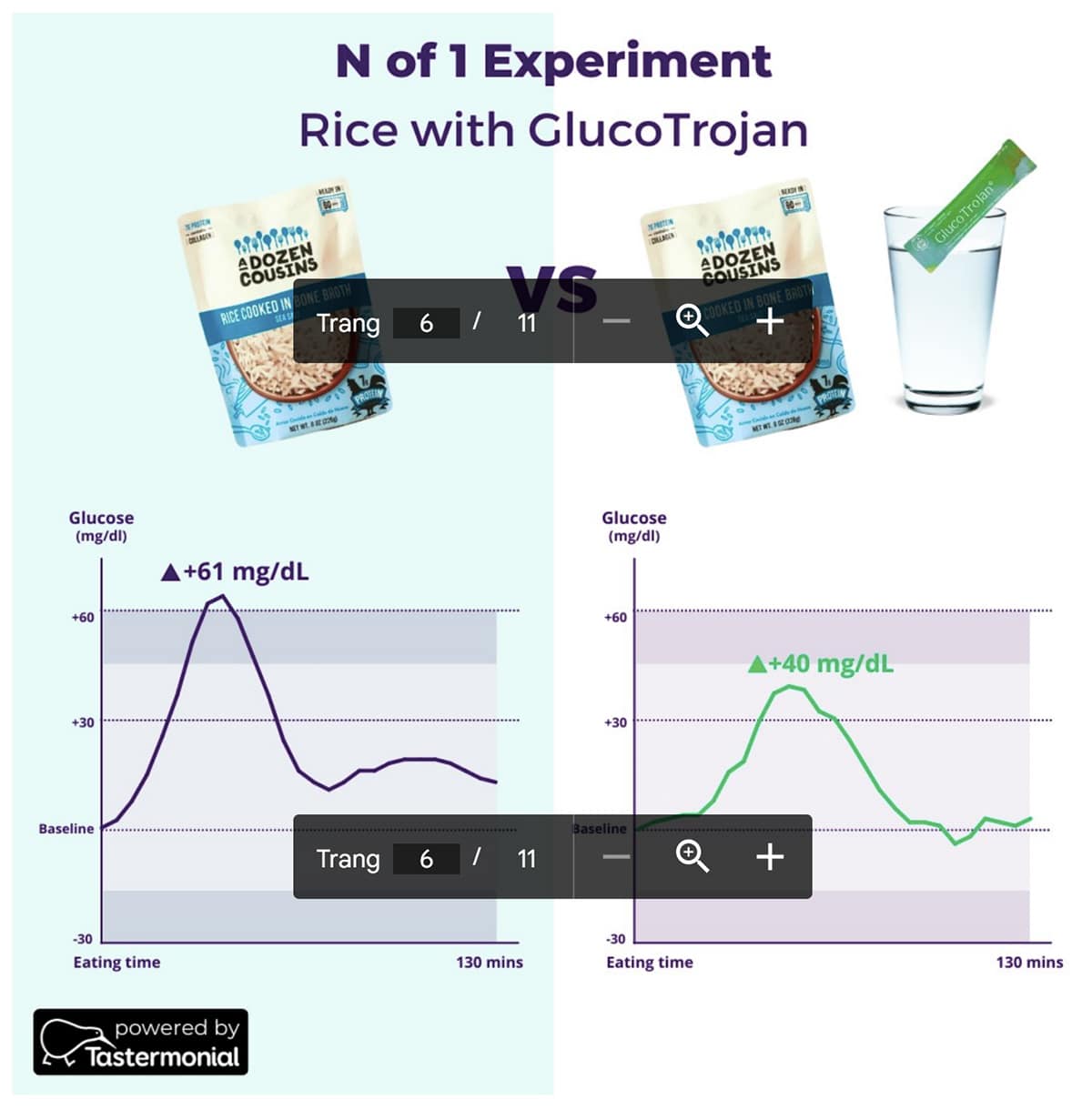
Our study showed that consuming rice with GlucoTrojan® significantly lowered the postprandial blood sugar levels by 26 ± 11 mg/dl compared to consuming rice only (P < .0005). A case example is shown in Fig 2: rice consumption alone spiked the blood glucose levels by 61 mg/dl, which remained elevated even after 2 hours , indicating impaired insulin sensitivity. Consuming rice with GlucoTrojan®, however, mitigated this surge by 21 mg/dl, allowing the blood glucose levels to return to baseline faster (Fig 2).
We also found that GlucoTrojan® reduced the iAUC (incremental area under the curve) by 1166 ± 610, equivalent to a 31% decrease in the rice’s glycemic index. iAUC is the gold standard method for measuring glycemic index, a measure of how fast carbohydrates elevate blood sugar levels. Foods with lower glycemic index undergo slower digestion and absorption processes, allowing better control of blood glucose and insulin response [21]. Importantly, such benefits of GlucoTrojan® were observed in 84-88% of the participants (Fig 3).
Overall, GlucoTrojan® offers a safe and convenient way to optimize glucose metabolism in individuals who seek to improve their health and well-being. Consumers of GlucoTrojan® can expect a less drastic spike in blood glucose and insulin levels after meals, promoting increased satiety, enhanced insulin responsiveness, and more efficient cellular energy utilization.
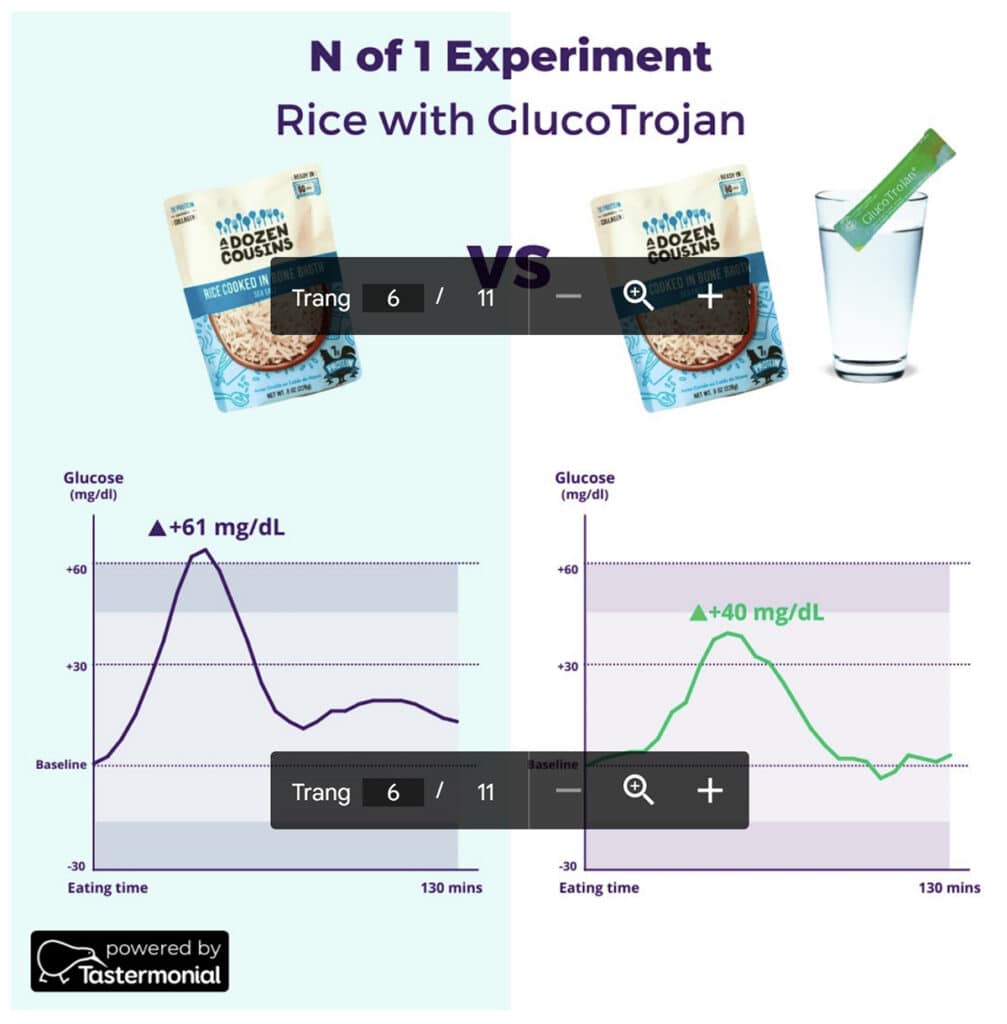
Figure 3. Peak glucose concentration (mg/dl) and iAUC value of healthy individuals with prediabetes after consuming rice with versus without GlucoTrojan®.
GlucoTrojan®: More Than Just Improved Blood Glucose Response
Besides mulberry extract, GlucoTrojan® formulation also contains Mangifera indica (mango)
leaf extract and banana stem juice extract, which benefit consumers’ health as well.
Mango leaves are rich in phytochemicals (e.g., mangiferin, phenolic acids, and carotenoids) and have been widely studied for their various health benefits, such as neuroprotective, cardioprotective, and anti-diabetic effects [22, 23]. For instance, mangiferin and mango leaf extract have been found to strengthen neural connections in the brain and enhance cognitive function in healthy individuals in RCT [24]. Moreover, the anti-inflammatory and antioxidant effects of mangiferin have been shown to reduce cardiac tissue injury in rats with diabetic cardiomyopathy [25]. Similar to DNJ in mulberry extract, mango leaf extract also has the capacity to inhibit α-glucosidase enzymes and decrease glucose absorption [26].
Banana stem juice is traditionally used to prevent and treat kidney stones and diabetes. Recent reports have confirmed such medicinal properties of banana stem juice extract, owing to its rich content of antioxidant phytochemicals, such as chlorogenic acid and triterpenoids [27, 28]. For instance, banana stem juice extract has been shown to reduce postprandial glucose and lipid levels, resulting in an improved diabetic condition in rats [29].
Therefore, GlucoTrojan® is formulated with three synergistic ingredients capable of improving blood glucose response after meals, contributing to numerous health benefits down the line. Research indicates that a minimal spike in postprandial blood glucose levels promotes satiety and prevents ‘sugar crash’ and subsequent cravings, helping us to manage our waistline more easily [30]. Healthier glucose response also prevents insulin resistance and even allows cells to be re-sensitized to insulin, protecting against the development of diabetes and obesity [31].
Moreover, enhanced insulin sensitivity is a prime indicator of overall health. As a result of insulin resistance, unregulated surges in blood glucose catalyze the formation of advanced glycation end-products (AGEs) – proteins or lipids that undergo glycation and oxidation in the presence of excess sugar [32]. AGEs accumulation accelerates aging and other age-related diseases, such as cardiovascular, neurodegenerative, and cancer diseases [33].
Ultimately, the mechanism of GlucoTrojan® in modulating blood glucose spikes after meals protects against not only diabetes and obesity but a myriad of other diseases as well. Elevate your pursuit of optimal health today by starting with GlucoTrojan®. Do not just aspire to live healthier; actualize it with the proven benefits of GlucoTrojan®,
Reference list
- Zimmet, P.Z., Diabetes and its drivers: the largest epidemic in human history? Clin Diabetes Endocrinol, 2017. 3: p. 1.
- The Centers for Disease Control and Prevention. Adult Obesity Facts. 2022 [cited 2023 16 September]; Available from: https://www.cdc.gov/obesity/data/adult.html.
- The Centers for Disease Control and Prevention. By the Numbers: Diabetes in America. 2022 [cited 2023 16 September]; Available from: https://www.cdc.gov/diabetes/health equity/diabetes-by-the-numbers.html.
- Temelkova-Kurktschiev, T. and T. Stefanov, Lifestyle and genetics in obesity and type 2 diabetes. Exp Clin Endocrinol Diabetes, 2012. 120(1): p. 1-6.
- DiNicolantonio, J.J. and A. Berger, Added sugars drive nutrient and energy deficit in obesity: a new paradigm. Open Heart, 2016. 3(2): p. e000469.
- Kahn, S.E., R.L. Hull, and K.M. Utzschneider, Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature, 2006. 444(7121): p. 840-6.
- Hostalek, U., Global epidemiology of prediabetes – present and future perspectives. Clin Diabetes Endocrinol, 2019. 5: p. 5.
- Macdonald, I.A., A review of recent evidence relating to sugars, insulin resistance and diabetes. Eur J Nutr, 2016. 55(Suppl 2): p. 17-23.
- Ramappa, V.K., et al., Mulberry 1-deoxynojirimycin (DNJ): an exemplary compound for therapeutics. The Journal of Horticultural Science and Biotechnology, 2020. 95(6): p. 679-686.
- Li, Y.G., et al., 1-deoxynojirimycin inhibits glucose absorption and accelerates glucose metabolism in streptozotocin-induced diabetic mice. Sci Rep, 2013. 3: p. 1377.
- Mudra, M., et al., Influence of mulberry leaf extract on the blood glucose and breath hydrogen response to ingestion of 75 g sucrose by type 2 diabetic and control subjects. Diabetes Care, 2007. 30(5): p. 1272-4.
- Asai, A., et al., Effect of mulberry leaf extract with enriched 1-deoxynojirimycin content on postprandial glycemic control in subjects with impaired glucose metabolism. J Diabetes Investig, 2011. 2(4): p. 318-23.
- Lown, M., et al., Mulberry-extract improves glucose tolerance and decreases insulin concentrations in normoglycaemic adults: Results of a randomised double-blind placebo controlled study. PLoS One, 2017. 12(2): p. e0172239.
- Riche, D.M., et al., Impact of mulberry leaf extract on type 2 diabetes (Mul-DM): A randomized, placebo-controlled pilot study. Complement Ther Med, 2017. 32: p. 105108.
- Wang, R., et al., Mulberry leaf extract reduces the glycemic indexes of four common dietary carbohydrates. Medicine (Baltimore), 2018. 97(34): p. e11996.
- Thaipitakwong, T., et al., A randomized controlled study of dose-finding, efficacy, and safety of mulberry leaves on glycemic profiles in obese persons with borderline diabetes. Complement Ther Med, 2020. 49: p. 102292.
- Thondre, P.S., et al., Mulberry leaf extract improves glycaemic response and insulaemic response to sucrose in healthy subjects: results of a randomized, double blind, placebo controlled study. Nutr Metab (Lond), 2021. 18(1): p. 41.
- Gheldof, N., et al., Effect of Different Nutritional Supplements on Glucose Response of Complete Meals in Two Crossover Studies. Nutrients, 2022. 14(13).
- McCarthy, C.M., Randomized controlled trials. Plast Reconstr Surg, 2011. 127(4): p. 1707-1712.
- Bansal, N., Prediabetes diagnosis and treatment: A review. World J Diabetes, 2015. 6(2): p. 296-303.
- Vlachos, D., et al., Glycemic Index (GI) or Glycemic Load (GL) and Dietary Interventions for Optimizing Postprandial Hyperglycemia in Patients with T2 Diabetes: A Review. Nutrients, 2020. 12(6).
- Kumar, M., et al., Mango (Mangifera indica L.) Leaves: Nutritional Composition, Phytochemical Profile, and Health-Promoting Bioactivities. Antioxidants (Basel), 2021. 10(2).
- Zivkovic, J., et al., Pharmacological properties of mangiferin: bioavailability, mechanisms of action and clinical perspectives. Naunyn Schmiedebergs Arch Pharmacol, 2023.
- Lopez-Rios, L., et al., Central nervous system activities of extract Mangifera indica L. J Ethnopharmacol, 2020. 260: p. 112996.
- Hou, J., et al., Mangiferin suppressed advanced glycation end products (AGEs) through NF kappaB deactivation and displayed anti-inflammatory effects in streptozotocin and high fat diet-diabetic cardiomyopathy rats. Can J Physiol Pharmacol, 2016. 94(3): p. 332-40.
- Kulkarni, V.M. and V.K. Rathod, Exploring the potential of Mangifera indica leaves extract versus mangiferin for therapeutic application. Agriculture and Natural Resources, 2018. 52(2): p. 155-161.
- Panigrahi, P.N., et al., Antiurolithiatic and antioxidant efficacy of Musa paradisiaca pseudostem on ethylene glycol-induced nephrolithiasis in rat. Indian J Pharmacol, 2017. 49(1): p. 77-83.
- Abu Zarin, M., et al., Investigation of potential anti-urolithiatic activity from different types of Musa pseudo-stem extracts in inhibition of calcium oxalate crystallization. BMC Complement Med Ther, 2020. 20(1): p. 317.
- Dikshit, P., et al., Antidiabetic and antihyperlipidemic effects of the stem of Musa sapientum Linn. in streptozotocin-induced diabetic rats. J Diabetes, 2012. 4(4): p. 378-85.
- Mantantzis, K., et al., Sugar rush or sugar crash? A meta-analysis of carbohydrate effects on mood. Neurosci Biobehav Rev, 2019. 101: p. 45-67.
- Aller, E.E., et al., Starches, sugars and obesity. Nutrients, 2011. 3(3): p. 341-69.
- Goldin, A., et al., Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation, 2006. 114(6): p. 597-605.
33. Prasad, C., et al., Advanced Glycation End Products and Risks for Chronic Diseases: Intervening Through Lifestyle Modification. Am J Lifestyle Med, 2019. 13(4): p. 384-404.