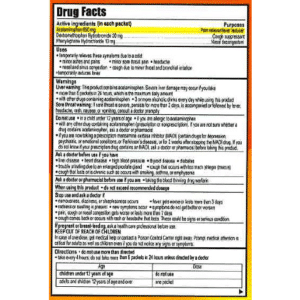
Active IngredientsPurposesCalcium carbonate 500 mg………………………………………………Antacid WarningsDo not use if you have ever had an allergic reaction to this product or any of its ingredients. Ask a doctor or pharmacist before use if you are presently taking a prescription drug. Antacids may interact with certain prescription drugs. When using this product
If pregnant or breast-feeding, ask a health professional before use. Keep out of reach of children. Uses for the relief of
Directions
Other information
Inactive IngredientsDextrose monohydrate, Saccharin sodium, Sucrose, Dokudami extract, FD&C Blue No. 1, FD&C yellow No. 5, Povidone K30, Water, Xylitol, Sorbitol, Magnesium Stearate, Peppermint, Silicon Dioxide Affordable Quality Pharmaceuticals, Garden Grove, CA 92841, USA |









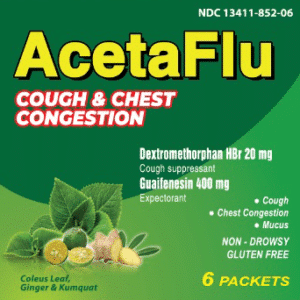







Reviews
There are no reviews yet.